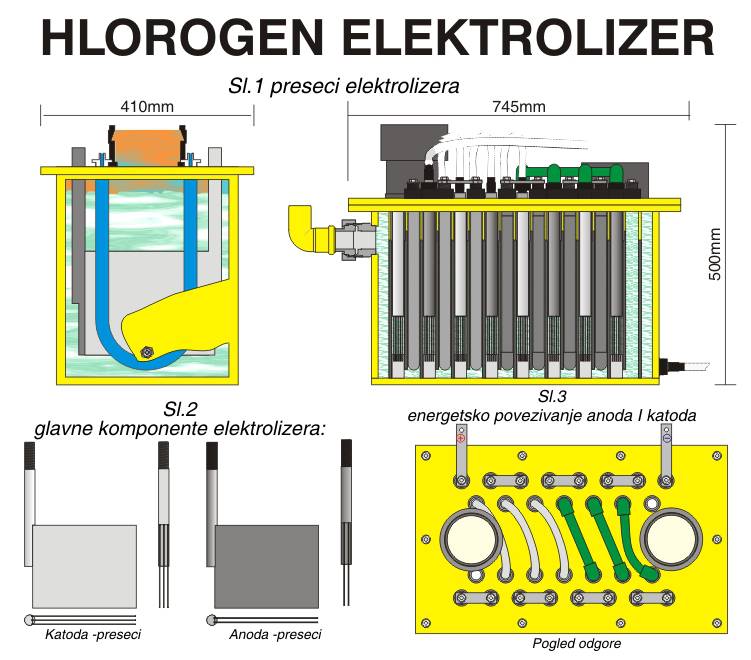
Hlorogen electrolyzers are modular type with serial connected cells in number wich depends on capacity.
Each cell contains two titanium anodes and three Hastelloy cathodes.
The electrodes are activated with a precious metal oxide, they have high ion-selectivity and corrosion stability.
Electrolyser type is «filter press» with a plastic case. Anodes are manufacturer from the modified titanium with a precious metal oxide coating specially develop for usage in the electrolytic process with diluted brine solution with efficiency of up to 98%. Cathodes are made from the stainless steel («Hastelloy» alloy) with a special coating, which allows electrical power efficiency above 85% for hydrogen separation reaction, which brings high Sodium-chlorite to the Sodium-hypochlorite conversion rate.
Electrolyser converts diluted brine (3%) into diluted (1%) sodium hypochlorite solution with no chlorine in the air.
In the electrolysis process a chlorine is separated at the anode and hydrogen at the cathode. Chlorine then reacts with the electrolyte and form sodium hypochlorite. Produced sodium hypochlorite solution is transferred in the sodium hypochlorite tank. Separated hydrogen is mixed with the air and released in the atmosphere through ventilation system.
In it’s lover part, the electrolyzer has temperature sensor to stop the electrolysis process when temperature of the electrolyte reach it’s maximum.